What Is the Key Word Describing a Covalent Bond
What is the difference between Covalent and Ionic Bonds. What happens to the stability of atoms when they form covalent bonds.
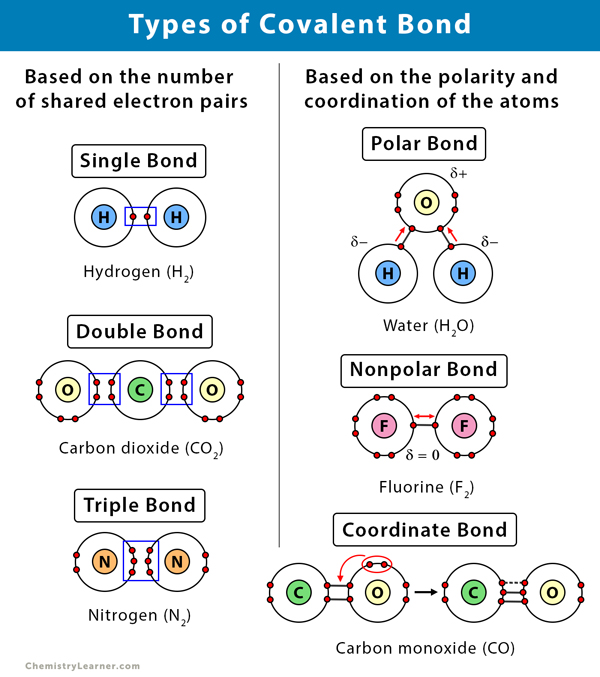
Covalent Bond Definition Types And Examples
The covalent bond is also called a molecular bond.

. A molecule is the smallest unit of a covalent compound. A dative bond is also termed as Coordinate Covalent bond. The reaction components of covalent bonds are electrically neutral whereas for ionic bonds they are both.
A covalent bond forms when two non-metal atoms share a pair of electrons. Ionic bonds occur through the interaction between cations and anions. Covalent bonds form between two nonmetals.
Ionic bonds result from the transfer of electrons from one atom to another. An atom that shares one or. Ionic bonds occur when the atoms are electrostatically attracted towards each other.
The forces of attraction or repulsion between two atoms when they share electron pair or bonding pair is. Covalent bonds occur through the interaction of neutral atoms. In a covalent bond the atoms are bound by shared electrons.
Tap card to see definition. Formed by SHARING valence electrons to fill outer shell octet rule. Chemical bonding section 6-1 short answer answer the following questions in describe how a covalent bond holds two atoms chapter 6 review.
A covalent bond is a chemical bond in which pairs of electrons are shared between two atoms. Covalent bonds usually form between non-metal atoms. Contains two or more NON-METAL atoms.
Covalent Bonding Worksheet Key Chemistry Covalent Bonding Worksheet Name Date 1 N2 7 Hcl 2 H2o 8 Ch3oh 3 Co2 9 H2s 4 Nh3 10 I2 5 Ch4 11 Chcl3 6 So3 Course Hero. What holds a covalent bond together. 100 1 rating Covalent bonds are formed by sharing of electrons having similar.
Diagram pairs of atoms that can form single double and triple bonds. Sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell which is. Click card to see definition.
At the centre of the atom neutrons and protons stay together. Non-metal non-metal covalent bond. A molecule is a group of two or more atoms joined together by covalent bonds.
Usually an electron is. Molecules of the same element or compound always contain the same number of atoms of each element. Draw a graph showing the change in potential energy when atoms form covalent bonds.
-In a covalent bond the atoms are bound by shared electrons. What is a covalent bond. A line can be used to represent a covalent bond between two atoms.
In a true covalent bond the electronegativity values are the same eg H 2 O 3 although in practice the electronegativity values just need to be close. A chemical bond that is formed between two atoms due to sharing of the electron pair in which only one atom provides a shared pair of electron for bond formation. If the electron is shared equally between the atoms forming a covalent bond then the bond is said to be nonpolar.
What type of atoms form covalent bonds. Describe an ionic bond in your own words. The electrons involved are in the outer shells of the atoms.
Strength of a bond increases as. The covalent bonds are also termed as molecular bonds. -In an ionic bond the atoms are bound together by the attraction between oppositely-charged ions.
It is that type of chemical bond in which one atom provides a shared pair of electron for the formation of a bond. The pair of electrons participating in this type of bonding is called shared pair or bonding pair. 2 Describe the relationship between the length of a bond and the strength of that bond.
Covalent bonding takes place where the electrons are shared between the atoms involved in the formation. The attractions between the shared electrons and protons in. Click again to see term.
A chemical bond formed when two or atoms share electrons. Covalent bonds result from two atoms sharing electrons.

Covalent Bond Definition Properties Examples Facts Britannica

No comments for "What Is the Key Word Describing a Covalent Bond"
Post a Comment